Inspirating Info About How To Find Out Protons

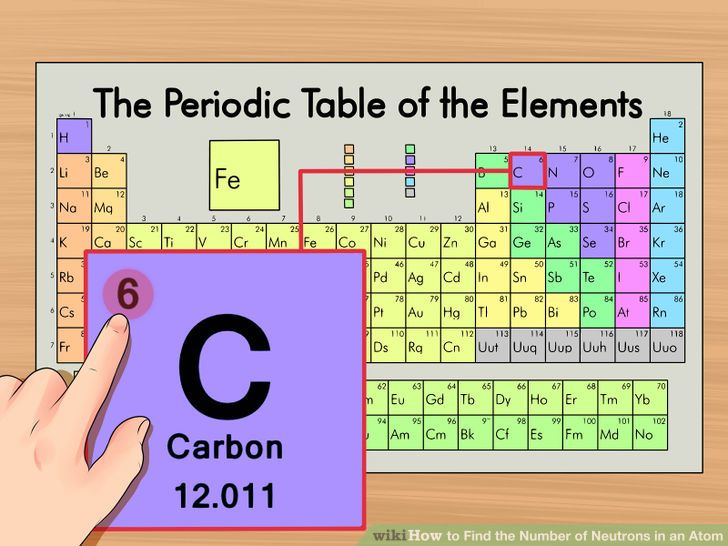
Find your element on the.
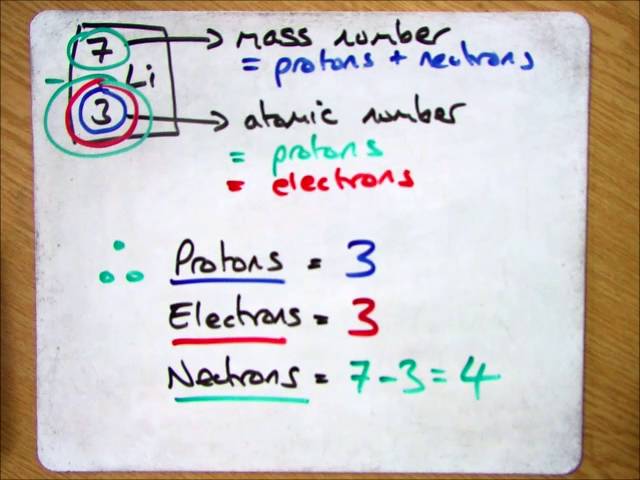
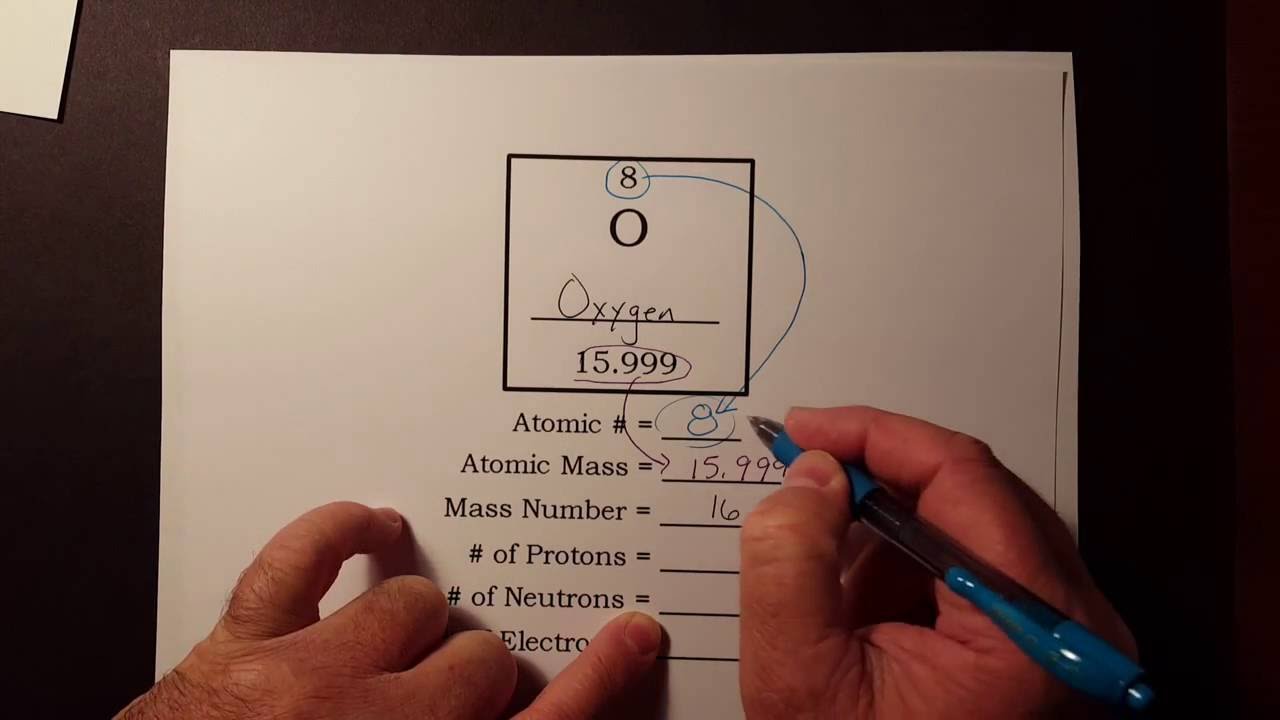
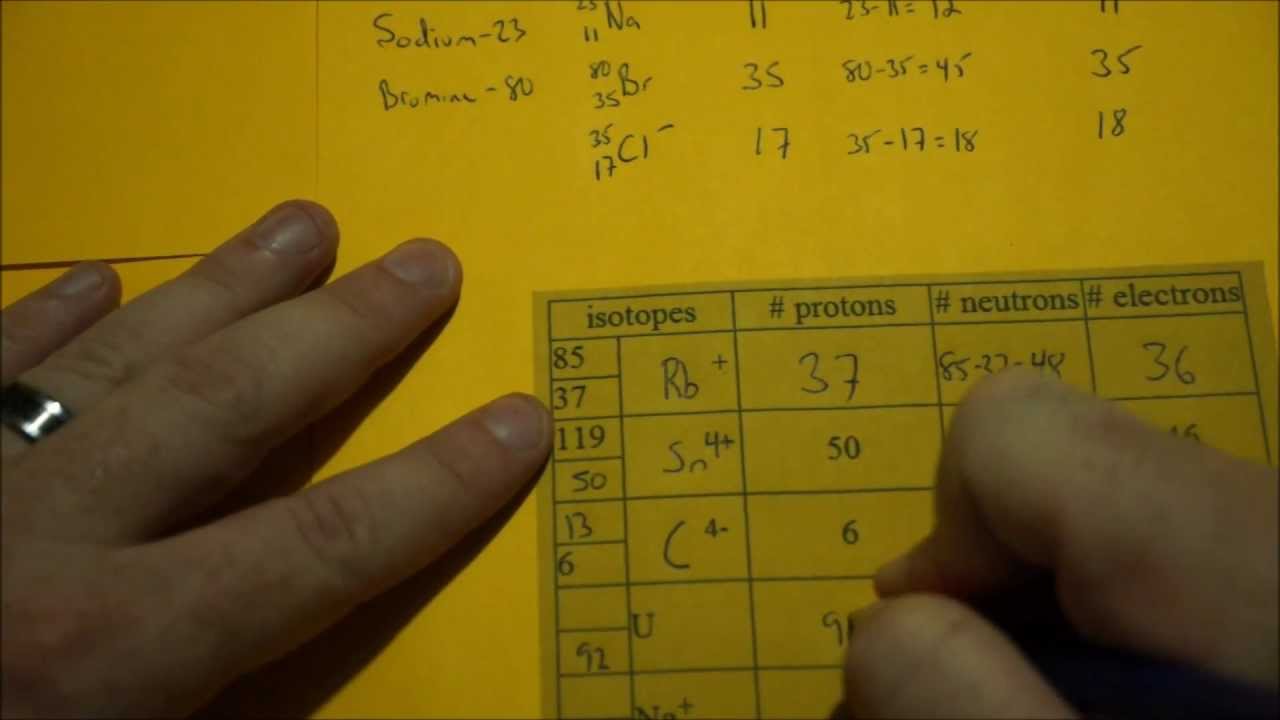
How to find out protons. Number of electrons = number of protons = atomic number. # of protons = atomic number. The number of electrons in a neutral atom is equal to the number of protons.
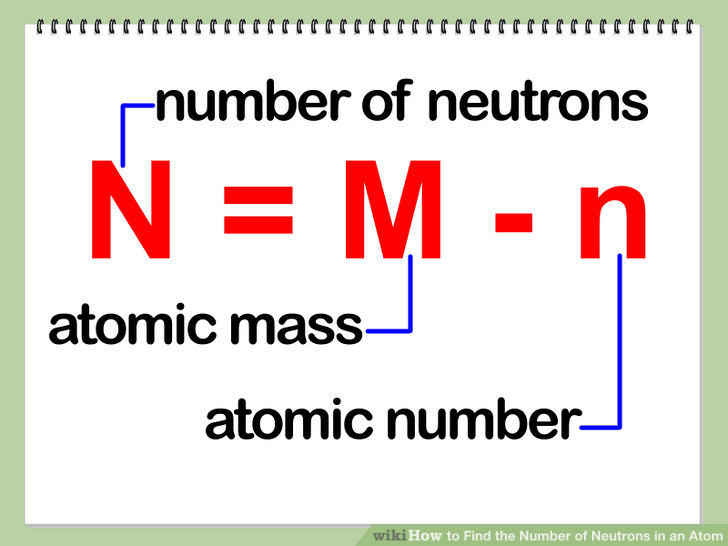
It also explains the differe. From the periodic table we can find. We know that an equal number of protons of atomic number are located in the nucleus of the element.
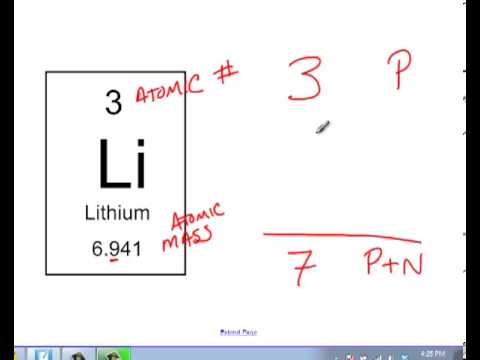
In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element carbon (c). Oxygen has an equal to ##8##, so it will have ##8## protons in its nucleus. The number of protons is equal to the number of electrons and the atomic number.
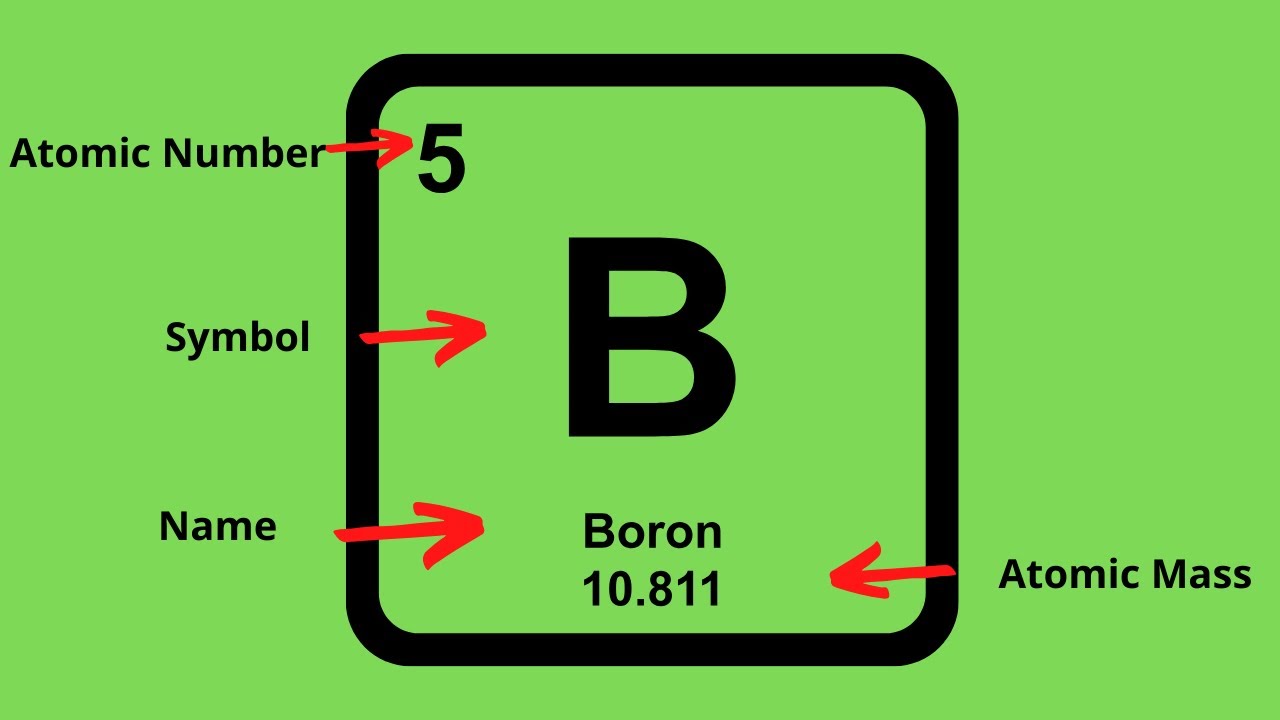
The periodic table is a chart that organizes elements by their atomic structure. Number of protons = the. For any atom, what you.
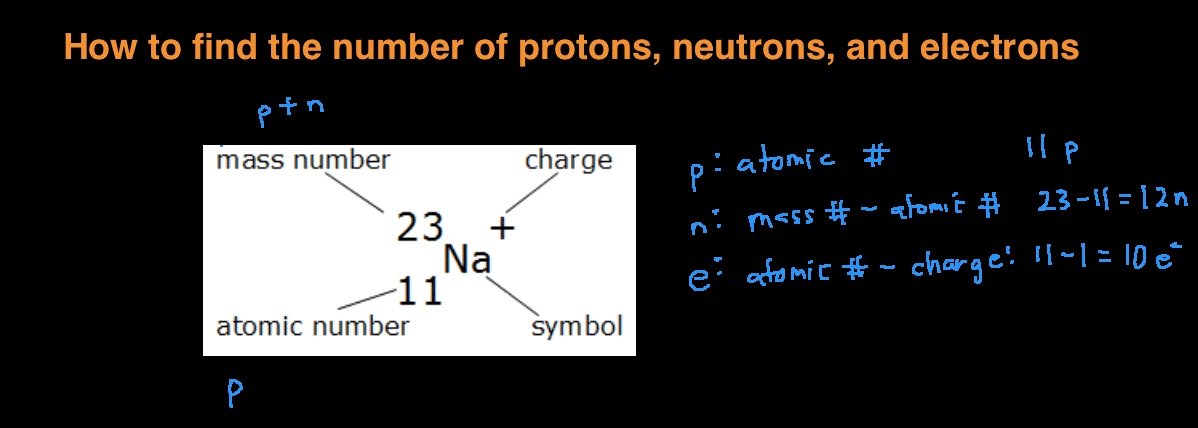
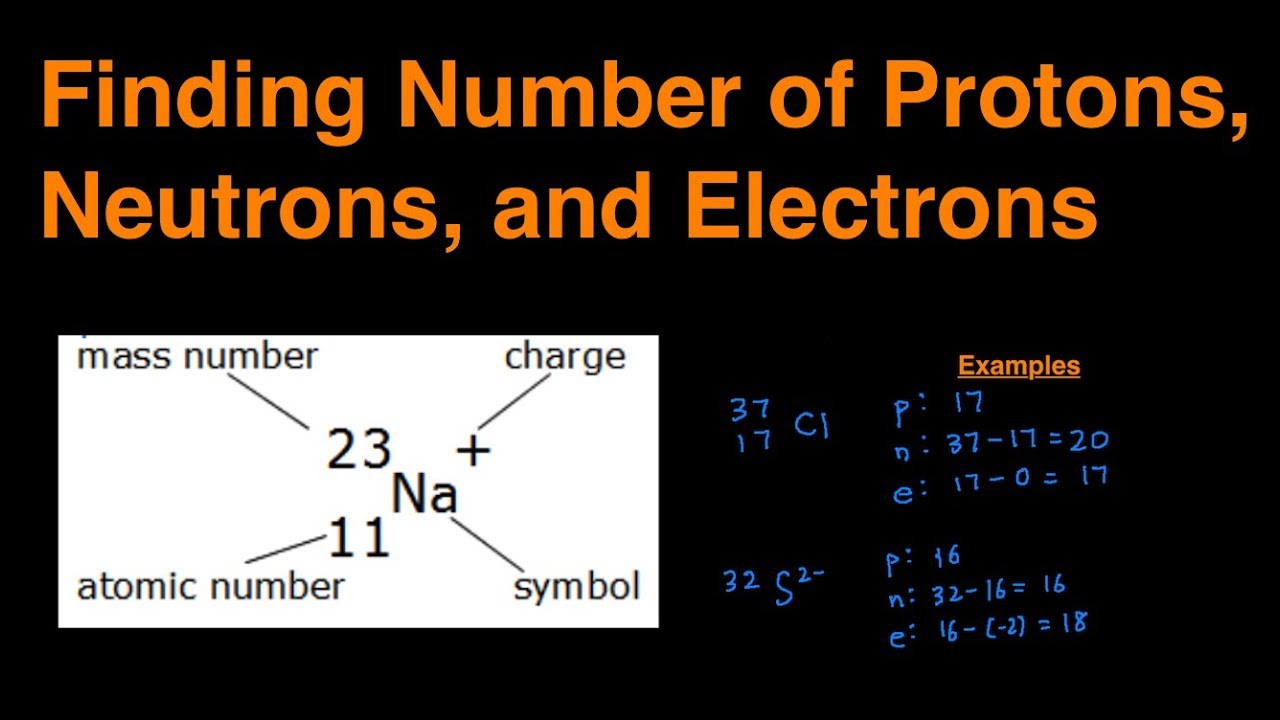
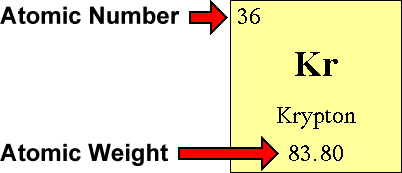
The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square. Rules to finding number of protons, neutrons, and electrons. To know the number of electrons, you need to know the atomic number of that element.
Number of protons = atomic number number of electrons = number of protons = atomic number number of. Finding the number of protons. Solution use the periodic table to find the atomic number of sc ( scandium ).